BIA-ALCL
Breast implant associated anaplastic large cell lymphoma (BIA-ALCL) is a cancer of the immune system caused by breast implants. It is generally found in fluid collection in between the implant and capsule, in a seroma, or in a nodule in the capsule. Physical signs are effusion, swelling, pain, inflammation, mass, ulceration, and others. The overwhelming symptoms in a majority of patients is a delayed seroma, persistent swelling, and pain. While even more rare some patients may present skin changes (rashes), lymphadenopathy, capsular contracture, or a potentially palpable mass.1 CD30 is the diagnostic test being used to distinguish BIA-ALCL. It is widely accepted that there is a direct correlation between textured implants and the development of BIA-ALCL. As of March 2019, there have been no confirmed cases of a BIA-ALCL in a patient with smooth only device, that is not to say it will not eventually happen.
Risks
“The current lifetime risk of BIA-ALCL is estimated to be 1:2,207 – 1:86,029 based upon variable risk with different manufacturer types of textured implants.” – American Society of Plastic Surgeons (ASPS), October 25, 2019.
Updates
As of January 2024, the American Society of Plastic Surgeons (ASPS) recognizes 1,355 cases worldwide. This is based on a global network of international plastic surgery societies collaborating to share and track cases.
The PROFILE registry is a joint collaboration between the FDA and ASPS/PSF to track BIA-ALCL patients. The ASPS reports there have been 428 suspected/confirmed U.S. cases reported to the registry. These are included in the total 1,355 cases worldwide.
In March 2019, the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE), Initial Report of Findings, 2012-2018 was published with a summary of the PROFILE registry’s findings.
FDA adverse event reports are another form of BIA-ALCL tracking. On February 6, 2019, the FDA released an update (FDA Summary of Raw MDR Data Received as of September 30, 2018) stating that as of September 2018, the FDA received a total of 660 medical device reports (MDRs) of BIA-ALCL – however after removing duplicates – 457 are considered unique BIA-ALCL reports. On August 20, 2020, the FDA released an update on adverse events reported to the Agency with 733 unique confirmed cases worldwide including 36 deaths, see FDA Medical Device Reports of BIA-ALCL.
Sample of FDA Adverse Event Reports on BIA-ALCL:
Join the Facebook group ALCL in Women with Breast Implants BIA-ALCL to view reports by country. The FDA MAUDE database contains adverse event reports involving medical devices. It includes voluntary, user facility, distributor, and manufacturer reports. Click here to see a sample of seven MAUDE BIA-ALCL reports submitted to the FDA.
Note: Parentheses represent redacted information to protect privacy.
1. Company rep reported right side anaplastic large-cell lymphoma and “subcutaneous nodules and lymph nodes. ” the pt had a bilateral reconstruction seven years ago with style 410 breast implant placed on the left side and a style 115 placed on the right side. The pt had done well until she presented last week with a pathology report from her oncologist stating that she had alcl. The pt stated that she had nodules on the right axilla. A pet scan was carried out that showed metastasis in the lung and bone marrow involvement. No seroma was noted. The oncologist has decided on her treatment plan to exclude radiation. Explant surgery will take place (b)(6) 2013. (Reported in 2013, Allergan silicone) Link.
2. Anaplastic large cell lymphoma of the breast arising around mammary implant capsule: an (b)(6) report written in aesthetic plastic surgery 2013 reports alcl, seroma, pain. Additional information noted in article anaplastic large cell lymphoma of the breast arising around mammary implant capsule: an italian report written in aesthetic plastic surgery 2013 article notes in regards to the right side, “necrosis and chronic inflammation signs are present” and “skin above the implant became red and painful and the patient had febrile episodes. ” treatment noted for the event of seroma as “a broad-spectrum antibiotic. ” (Reported in 2013, Allergan silicone) Link.
3. Healthcare professional reports a case of lymphoma and other b-symptoms via mw (b)(4) the mw notes that: “the reporter called on behalf of a pt who was diagnosed with alcl. The pt presented with anaplastic large cell lymphoma, diagnosed in 2013. History of hodgkin’s lymphoma diagnosed in 2011. These two events came about after the pt underwent breast augmentation in 1994. In 2010, pt presented with an abnormal mammogram performed in 2010. Breast pain, skin color change, skin texture change, and inflowing diffusion form the right breast up to right neck and shoulder. The pt was running a fever throughout the entire process. After an mri and subsequent test, the pt was diagnosed with hodgkin’s lymphoma and underwent mantle radiation. In 2012, the pt underwent surgery essentially for a breast mass, but the pt also desired a mastectomy for removal of right and left implants and capsules. The pathology of the operation soon reported that the pt also has alcl; the mass had come from the lymphoma. ” (Reported in 2013, Allergan saline) Link.
4. Pt is a female who underwent left mastectomy in 1996, for ductal carcinoma in situ with tissue expanders and saline implant reconstruction. She presented in 2010, with a peri-implant hematoma, though possibly post-traumatic. She underwent evacuation of the hematoma and change to a silicone gel implant. All pathology specimens were negative for tumor. She again presented in 2012, with a spontaneous hematoma and at surgery multiple biopsies revealed anaplastic large cell lymphoma (alcl) limited to the periprosthetic capsule and hematoma fluid. After an extensive hematologic and metastatic workup which was negative, she underwent removal of the implant and total periprosthetic capsulectomy. Capsular pathology showed alcl. (Reported in 2012, Mentor silicone) Link.
5. On (b)(6) 2010, diagnosed with anaplastic large cell lymphoma (alcl) alk-negative. Possibly related or caused by breast implants received in (b)(6) 2002 for augmentation. Experienced complications with left implant diagnosed as capsular contraction. Implant replaced on (b)(6) 2008. Still experiencing capsular contraction after replacement. (b)(6) 2010 – (b)(6) 2011: received 12 doses of chemotherapy, received 20 doses of radiation therapy. Preparing for stem cell transplant scheduled for (b)(6) 2011. (b)(6) 2010: needle biopsy – diagnosis lymphoma. (b)(6) 2010: surgical biopsy – diagnosis alcl. (b)(6) 2010: surgical biopsy – diagnosis alcl. (Reported in 2011, Allergan saline) Link.
6. The original purchase date of this device was (b)(6)2004. In (b)(6) 2006, the pt was implanted with mentor siltex saline devices during a revision augmentation procedure. In (b)(6) 2008, the devices were replaced with mentor smooth saline devices due to a left device deflation. In (b)(6) 2010, the pt had both implants removed due to recurring fluid accumulation in the right breast. On (b)(6)2010, the pt was diagnosed with alcl (t-cell lymphoma). No further info is available at this time. (Reported in 2010, Mentor saline) Link.
7. It was reported by a physician that a (b)(6) year old female patient was diagnosed with alcl on (b)(6) 2017. This patient¿s medical history includes diagnosis of left breast invasive ductal carcinoma in (b)(6) 2015. She underwent bilateral mastectomy and bilateral tissue expander placement in (b)(6) 2015. The patient had mentor tissue expanders that were implanted from (b)(6) 2015. The patient then had mentor memory shape low high moderate plus profile breast implants (catalog #334-1507, r. Side serial # (b)(4)) implanted in (b)(6) 2015. On (b)(6) 2017, the patient experienced a large right breast effusion that developed over 24-48 hours. The effusion was aspirated and tested using flow cytometry and cd30 ihc and came back positive for bia-alcl on (b)(6) 2017. The time between patient signs/symptoms of peri-implant alcl to definitive diagnosis was 1 week. The patient did not have any complications such as infection, hematoma, or implant rotation during implant course prior to alcl diagnosis. The patient did not experience skin lesions, fevers, night sweats or weight loss. There was no pain, redness, palpable breast mass, or capsular contracture. The lymphoma cells were found in the seroma fluid surrounding the implant. Immunohistochemical and flow cytometry testing showed alk negative and cd30 positive results. This is a pathologically confirmed stage ie primary diagnosis of alcl. Based on histology, there is no capsular involvement. The lymphoma cells were found in the effusion fluid surrounding the implant. The patient underwent bilateral implant removal and capsulectomies with no implant replacement on (b)(6) 2017. The implants were intact and not ruptured upon removal. (Reported in 2017, Mentor Memory Shape Silicone) Link.
Smooth vs. Textured
1. In February 2019, ASPS By The Numbers, and What They Mean was updated by Dr. Mark Clemens:
“The recent FDA update of 457 reports included 24 (5%) smooth implant reports.
This is similar to last year’s update of 414 reports in 2018 including 30 (7%) smooth implants and 359 reports in 2017 including 28 (8%) smooth cases, and 258 patients which included 11 (4%) smooth implant reports in 2016.
Previously, the FDA noted that all smooth reports were either mixed textured implant cases histories or no clinical history available for review. For the first time, the FDA reports smooth only cases made available to them. The FDA reports that they are aware of smooth only cases however they warn that this information is “unverified” and potentially “inaccurate.” To date, no purely smooth-implant case of BIA-ALCL has ever been reported in any series, registry or case report with a detailed history. The FDA confirmed that BIA-ALCL is predominantly associated with textured implants.”
2. In March 2019, the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE), Initial Report of Findings, 2012-2018 was published and includes: “All patients had a history of a textured device; there were no patients who had a smooth-only device history.”
3. In March 2019, the American Society for Aesthetic Plastic Surgery (ASAPS) released a monthly newsletter, including a BIA-ALCL update:
“Recognizing that by the FDA’s own admission the MAUDE database has incomplete data and in a large percentage father reported cases, the implant type(s) are unknown, I contacted each US implant manufacturer for comment. Allergan, Mentor, and Sientra all independently verified that they cannot document a smooth only case of BIA-ALCL as of February 2019. In all current global registries and databases there are no documented smooth only cases.”
4. Additionally, in the Facebook BII Support Groups, there has not yet been a purely smooth implant case of BIA-ALCL. This is not to say that it is not possible, but that it has yet to be seen. Regardless of surface type, symptomatic women should be tested. The NCCN guidelines do not specify a surface requirement for testing. Any symptomatic woman that is denied testing should report their clinician to the appropriate medical board.
1997
In as early as 1997, lymphoma was first identified around breast implants in isolated cases. See the research article Breast Implants and Cancer. It was written that lymphomas and breast implants “deserve[d] further attention.” Unfortunately, it took nearly 20 more years for BIA-ALCL to be highlighted. Click here to read the Breast Implants and Cancer (1997) section on lymphomas.
There have been several reports of lymphomas occurring among women with silicone breast implants; these reports are of interest because of evidence that implants can affect T-cell-mediated autoimmune reactions. At least four cases of cutaneous T-cell lymphomas have been reported among women with silicone gel breast implants ( 95 , 96 ). In several cases, the skin lesions began as eruptions overlying the implants, while in another instance a ruptured implant led to the diagnosis of pre-Sézary syndrome, which subsequently progressed to Sézary syndrome (a form of cutaneous T-cell lymphoma), leading to the suggestion that persistent antigenic stimulation might have been involved ( 96 ). Another report ( 97 ) noted a follicular mixed lymphoma adjacent to a surrounding granulomatous response in a woman who had painful capsular contracture. An additional case has been reported of a primary effusion lymphoma developing between a silicone breast implant and its capsule in the absence of a mass lesion in a woman negative for the human immunodeficiency virus ( 98 ). The histologic dissimilarity of the cancers in these reports argues against a common mechanism. Nonetheless, the risk of lymphomas among women with implants deserves further attention, given the reported immunologic disturbances following silicone exposure.
New scientific article reconsiders the first case of BIA-ALCL may have been in 1996 and brings up questions on if earlier cases will be found.
Reconsideration of the first recognition of breast implant-associated anaplastic large cell lymphoma: A critical review of the literature – February 2020, Annals of Diagnostic Pathology
BIA-ALCL Causation Theories
The cause is still unknown but is actively being studied. Some researchers have theorized that biofilm (bacteria) contributes to lymphoma and others have thought the rough surface and chemicals in the implants irritate the immune system. Both theories rely on the presence of persistent inflammation, which means chronic activation of immune cells and particularly the T lymphocytes, which are white blood cells involved with BIA-ALCL.
Throughout the body, there are many diverse populations of bacteria that are both beneficial and harmful. In recent years, there has been an increased focus in characterizing bacteria and analyzing patterns of bacteria to understand the possible correlation between normal versus infectious/cancerous scenarios – especially in relation to breast cancer. What has been discovered is that similar to how the gut has its own microbiome of good and bad bacteria, the normal breast tissue and human milk also have their own microbiology that over time is influenced by factors such as dietary and sugar changes. The article “Microbiota of the Human Breast Tissue” delves into the various specific bacteria that were found in human breasts. Since breasts are not sterile, if a foreign object is placed inside the body, it will be colonized and infected.
Biofilm is bacteria that adheres to the surfaces of medical devices. It can result in a low grade chronic bacterial infection, chronic inflammation, and capsular contracture. Some bacteria produce acid as they grow and this reduces the pH of the surrounding environment. In the closed off space between the surface of the implant and the inner capsule surface, the bacteria coating the implant could form an acidic environment that contributes potentially to the breakdown of silicone. Australian researchers found that biofilm from capsular contracture cases was different from the biofilm identified on 26 implants from lymphoma patients. This brings biolfim to light as “a possible infectious contributing cause” for the lymphoma.
The chemicals used in the manufacturing process, which are neurotoxic and carcinogenic, are also believed to be playing a role in the development of lymphoma. As of 2019, all BIA-ALCL cases have been found with textured implants, there have been no documented smooth only cases. The roughness of the surface is triggering chronic inflammation. Textured implants were designed to keep the implants in place, thus, the capsules embed themselves on and around the textured surface. This creates an intimate, hand in hand connection between the scar tissue and chemically abrasive textured surface. Over time, this can lead to a direct abrasive irritation of the immune system, significantly affecting T cells.
It is interesting to note the connection between polyurethane coated implants and textured implants. Polyurethane coated implants were the first type of breast implant linked to cancer, and textured implants have now become the second type of breast implant linked to cancer – what they both have in common is a chemically abrasive fuzzy surface. Polyurethane implants were in production from about 1980 to when the manufacturer voluntarily withdrew them in 1991 due to significant safety concerns. These implants were the precursors to the textured breast implants since the textured surface was thought to be important in reducing capsular contracture and firmness, but the implant manufacturers could not use polyurethane so instead they created the textured surface currently manufactured today (since the mid-1990’s). This textured surface is also linked to an increased occurrence of forming double capsules (scar tissue surrounding the implants) and seromas, thereby going against its intended purpose.1,2
Genetics has also been brought up but requires further investigation. Two published reports showed a genetic predisposition (germ line mutation in JAK1 and STAT3 genes).
Current BIA-ALCL Researchers
- Dr. Mark Clemens, Dr. Roberto Miranda, Dr. Charles E. Butler (US)
- Dr. Suzanne Turner (UK)
- Dr. Anand Deva (Australia)
NCCN BIA-ALCL Protocol
Proper protocol is the National Comprehensive Cancer Network (NCCN) guidelines, the recognized standard in cancer care. Below is the updated January 2020 NCCN Guidelines for BIA-ALCL. It can be found on the NCCN Guidelines App, select T Cell Lymphomas, and find the algorithms on pages 20-24.

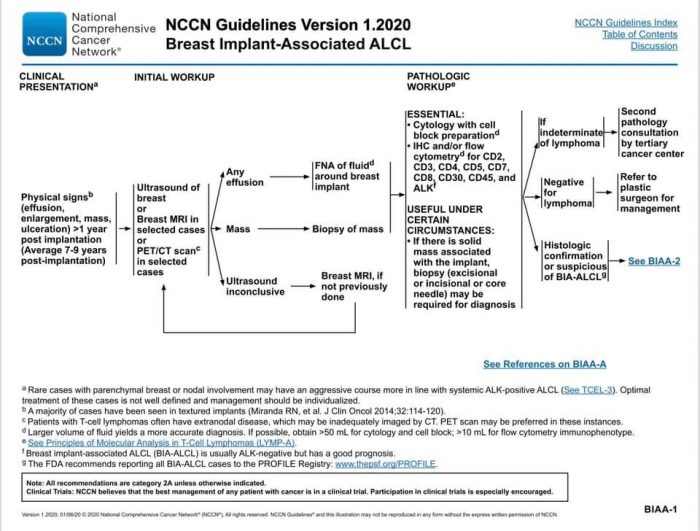

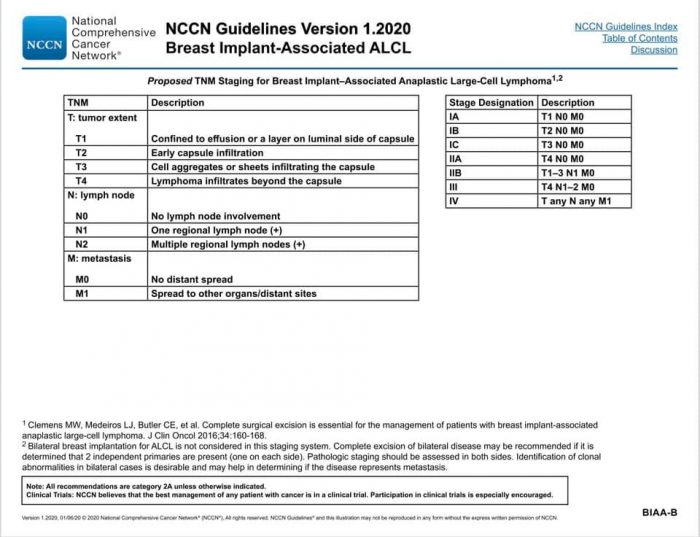
Credit: This information is being shared via the ALCL in Women with Breast Implants BIA-ALCL Facebook support group and their advocacy website, BIAALCL.com.
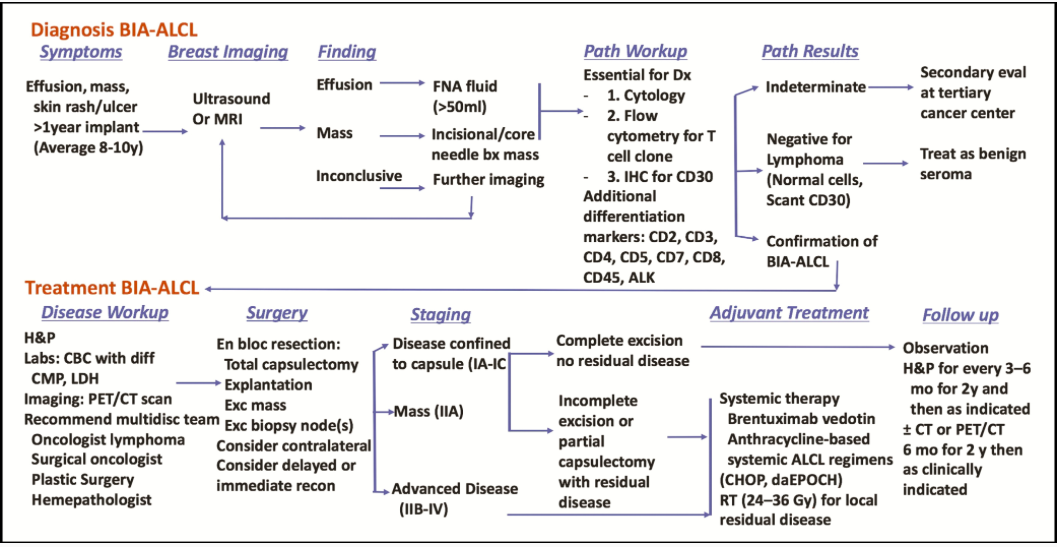
Figure 1. Breast implant-associated anaplastic large cell lymphoma disease algorithm.
Explant with Full Capsule Removal (En Bloc or Total Capsulectomy)
Very important to have full capsule removal with textured implants. There have been at least two cases of BIA-ALCL in the ALCL in Women with Breast Implants BIA-ALCL patient support group where women had textured implants removed but had capsules left in and were later diagnosed with BIA-ALCL.
Chemotherapy
The determination for chemotherapy is related to how much of the cancer is contained and able to be surgically removed versus if it is past the capsules and spreading. For some women the cancer is contained in-situ in a seroma (fluid collection) within the capsules or as a capsular tumor or palpable breast mass that can be surgically removed. For others it can be an infiltrative disease that cannot be surgically removed such as a mass outside the capsule, lymph node involvement (with or without breast mass) and rarely distant metastases – these cases would involve chemotherapy. Infiltrative disease may occur with or without a seroma. It’s not yet entirely understood if in-situ develops into infiltrative disease or if they are two distinct entities.
In 2018, an admin of the Facebook ALCL in Women with Breast Implants BIA-ALCL patient support group commented that about 30% of diagnosed women in the group needed chemotherapy in addition to removal of the capsules and implants. There are two main forms of chemotherapy available: CHOP (or CHOEP) and the more expensive Brentuximab. If women fail to respond to CHOP (about 50%), they then qualify for Brentuximab. The side effects of Brentuximab seem to be more tolerable and overall the preferred choice among women.
BIA-ALCL and Mammograms
There have been cases reported in the Facebook ALCL in Women with Breast Implants BIA-ALCL patient support group where mammograms have triggered breast swelling and led to BIA-ALCL diagnoses.
BIA-ALCL Documents to Share with Doctors
If you are symptomatic, having trouble getting answers or testing done, here are some documents to help educate doctors:
1) The most important is the NCCN Guidelines on the Diagnosis and Treatment of BIA-ALCL (2020)
2) FDA BIA-ALCL Letter to Healthcare Providers (2/6/19)
3) Aesthetic Society News (Winter 2018), pages 6, 7, and 9
4) Joint ASPS / ASAPS Statement
6) Diagnosis and Treatment of BIA-ALCL
8) ASPS Insurance Coverage for Third-Party Payers – BIA-ALCL. Addresses symptoms, insurance issues, insurance codes, imaging for evaluating symptoms, and more.
Credit: This information is being shared via the ALCL in Women with Breast Implants BIA-ALCL Facebook support group and their advocacy website, BIAALCL.com.
Click here to see a list of scientific articles.Breast Implant-Associated Anaplastic Large Cell Lymphoma: What We Know (2019). Link. Full article can be found in the ALCL in Women with Breast Implants BIA-ALCL Facebook support group
PSEN Breast Implant Associated Anaplastic Large Cell Lymphoma (2017). Link.
United States Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (2017). Link.
Breast implant associated Anaplastic Large Cell Lymphoma in Australia and New Zealand – high surface area textured implants are associated with increased risk (2017). Link.
Breast implant-associated anaplastic large cell lymphoma (2016). Link.
Diagnosis and Management of BIA-ALCL (2016). Link.
BIA-ALCL, The French National Cancer Institute Expert Opinion (2016). Link.
A further case report from the United Kingdom of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) and a reason to avoid the sub pectoral plane (2016). Link.
Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes (2016). Link.
Marked eosinophilia as initial presentation of breast implant-associated anaplastic large cell lymphoma (2016). Link.
- Scar capsule around breast implant must be removed if you want it insure you don’t have problems years later, including BIA-ALCL Here is a case of BIA-ALCL years after implants were removed.
MSCs and inflammation: new insights into the potential association between ALCL and breast implants (2016). Link.
Biomarkers Provide Clues to Early Events in the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma (2016). Link.
Whole Exome Sequencing Reveals Activating JAK1 And STAT3 Mutations In Breast Implant-Associated Anaplastic Large Cell Lymphoma Anaplastic Large Cell Lymphoma (2016). Link.
Axillary Lymphadenopathy: An Outstanding Presentation for Breast Implant-Associated ALK-Negative Anaplastic Large Cell Lymphoma (2015). Link.
Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinics-pathological entity (2010). Link.
Complete surgical excision is essential for management of patients with breast implant-associated anaplastic large-cell lymphoma (2016). Link.
Bacterial biofilm infection detected in breast implant–associated anaplastic large-cell lymphoma (2016). Link.
Marked eosinophilia as initial presentation of breast implant-associated anaplastic large cell lymphoma (2016). Link.
Breast implant-associated anaplastic large cell lymphoma: a systematic review (2015). Link.
Recurrent systemic anaplastic lymphoma kinase-negative anaplastic large cell lymphoma presenting as a breast implant-associated lesion (2015). Link.
The non-specific symptoms of breast implant-associated anaplastic large cell lymphoma resulting in delayed diagnosis: a case-based review (2015). Link.
CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia (2014). Link.
- The significant association of CD30 with EBV-positive non-GCB DLBCL suggests a distinct pathobiology for these cases.
Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients (2013). Link.
Implant-associated anaplastic large cell lymphoma of the breast: insight into a poorly understood disease (2013). Link.
Breast implant associated anaplastic large cell lymphoma: a case report and reconstructive option (2013). Link.
Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis (2013). Link.
Anaplastic large cell lymphoma, ALK-negative (2013). Link.
Chemotherapy-resistant breast implant-associated anaplastic large cell lymphoma (2013). Link.
Breast implant-associated ALCL: a unique entity in the spectrum of CD3o+ lymphoproliferative disorders (2012). Link.
Survival Signals and Targets for Therapy in Breast Implant–Associated ALK− Anaplastic Large Cell Lymphoma (2012). Link.
Primary anaplastic large-cell lymphoma associated with breast implants (2012). Link.
Anaplastic large cell lymphoma involving the breast: a clfinicopathologic study of 6 cases and review of the literature (2009). Link.
Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases (2009). Link.
- One case presented mastitis but was lymphoma
Primary anaplastic large cell lymphoma of the breast arising in reconstruction mammoplasty capsule of saline filled breast implant after radical mastectomy for breast cancer: an unusual case presentation (2009). Link.
Rare lymphoid malignancies of the breast: a report of two cases illustrating potential diagnostic pitfalls (2000). Link.
Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules (2016). Link.
Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-Cell lymphoproliferative disorder (2008). Link.
Malignant lymphoma and breast implants: a case of diffuse large b-cell lymphoma (DLBCL) with implant-near relapse localization (2010). Link.
Intravascular large B-cell lymphoma associated with silicone breast implant, HLA-DRB1*11:01, and HLA-DQB1*03:01 manifesting as macrophage activation syndrome and with severe neurological symptoms: a case report (2016). Link.
Anaplastic large-cell lymphomas of B-cell phenotype are anaplastic lymphoma kinase (ALK) negative and belong to the spectrum of diffuse large B-cell lymphomas (2000). Link.
Funding for Uninsured Patients
There is funding available for uninsured patients diagnosed with BIA-ALCL. It is funded by all three US manufacturers: Allergan, Mentor, and Sientra. It is for US patients only and a requirement is that the patient be diagnosed following the NCCN guidelines. This information is from the Aesthetic Society News Quarterly Newsletter of the American Society for Aesthetic Plastic Surgery, Spring 2018, page 54.

Report
If you have or have had breast implants and have been diagnosed with ALCL, please fill out the two reports below:
- FDA MedWatch Online Voluntary Reporting Form (for Adverse Reactions)
- Submit case report of BIA-ALCL to the PROFILE Registry to contribute to a better understanding of the causes and treatments of BIA-ALCL.
Resources
Facebook Support Groups
- ALCL in Women with Breast Implants BIA-ALCL and their advocacy website, BIAALCL.com.
- BIA-ALCL Clinician/Patient Discussion Group. Only for women diagnosed with BIA-ALCL and clinicians.
Government Health Agencies and Other
- FDA:
- FDA Updated 2019 Information:
- BIA-ALCL Letter to Healthcare Providers (2/6/19)
- DA Summary of Raw MDR Data Received as of September 30, 2018 (2/6/19)
- Statement from Binita Ashar, M.D., of the FDA’s Center for Devices and Radiological Health on agency’s continuing efforts to educate patients on known risk of lymphoma from breast implants (2/6/19)
- Canada: Canadian Society of Plastic Surgery on ALCL
- Australia: TGA on Breast Implants and Anaplastic Large Cell Lymphoma
- UK: MHRA on Breast Implants and Anaplastic Large Cell Lymphoma
Articles and Videos
- ASPS BIA-ALCL Physician Resources – The Numbers (Dr. Mark Clemens) (2/8/2019)
- ASPS BIA-ALCL Resources Q&A (7/30/2018)
- PSEN Breast Implant Associated Anapestic Large Cell Lymphoma (2017)
- Dr. Mark Clemens YouTube: “Patient Education BIA-ALCL“
- News Article: “Rare cancer reignites debate over breast implants’ safety.” (7/10/2017)
Advocacy
- BIAALCL.com
- BIA-ALCL Awareness – Just Call Me Ray Foundation (Non-Profit)
- Raylene Hollrah – Diagnosed with ALCL, story
Biopsy Testing (a list is currently pending and being created)
- Spectrum Women’s Imaging Centre – Sydney, Australia
Lawsuits
- There are BIA-ALCL lawsuits filed and more to come in the future. Attorney Dena Young Lebovic with Ross Feller Casey is currently filing these cases. Dena and co-counsel have 50+ BIA-ALCL cases filed in the New Jersey Superior Court. See Lawsuits for more information.
Hi Amanda Andrews, I know you are asking Linzi, but she wrote that post back in 2017 so I thought I’d answer incase she doesn’t get this.
I am also in the UK and was diagnosed with BIA-ALCL on the 16th January 2020… in Suffolk. The onset of very specific symptoms happened over a period of a few months. I got a raging rash on my right breast and it was my right breast that had the lymphoma. The breast was swollen and when an MRI was done it showed fluid between the implant and capsule. The only reason they would give me an MRI is, they believed I had a rupture and had told me to apply for special funding to get the implants removed. Whilst waiting for that funding they did an MRI. If you are in a lot of pain with your implants or, if you have a rupture or capsule contraction, then you can apply for funding to have them removed on the NHS.
Once I knew it was fluid, I knew there was a good chance of this being BIA-ALCL, so I told the breast surgeon, “before you operate, I need a needle aspiration and pathology done on this fluid”. Believe me, they didn’t want to do that. They just wanted to operate, which I now know would of likely killed me. I insisted but it was a horrible battle. They took the fluid and mocked me, telling me that I couldn’t possibly have BIA-ALCL. Talk about a turn around in their attitude when it came back positive for CD30. I sacked that surgeon and had a full oncological en-bloc by another NHS team.
I’m doing fine now and one year on, I’m in remission. Fortunately it was only in the fluid and hadn’t started to grow through the capsule. It is though, a high grade lymphoma, meaning, its a dangerous and fast growing lymphoma. Whilst still trapped between the implant and breast capsule, it has nowhere to go. This is why an oncological en-bloc is so important for people with a diagnosis. Sadly, not everyone gets diagnosed before surgery and that’s when it spreads quickly through the body.
I was poorly for about four years before being diagnosed with BIA-ALCL and looking thoroughly through my old medical notes, its obvious that there was a silent infection going on in my body during that sickness period. Every blood works I had done came back with raised white blood cells and CRP.
Also looking back, I realise that my breasts had slowly grown in size from when I first had the implants and that’s because they scar tissue was full of infected fluid. What eventually rang alarm bells was one breast suddenly went down in size. I thought I had a rupture and so did my breast specialist but that breast implant was perfectly fine. The other breast… the normal sized one, was full of fluid though. I was so puzzled. How could this much smaller breast be normal? On the needle aspiration I had done, it didn’t only come back CD30 positive but with a high grade infection. Before surgery I went on specific antibiotics and by the time I had surgery, my second breast was also a lot smaller than it had been for many years.
I don’t think doctors are looking at this with BII. I most definitely had all the symptoms of BII for four years prior to being diagnosed with lymphoma and it wasn’t lymphoma at that point, it was just an infection.
If you are diagnosed with Hodgkin’s lymphoma instead of non Hodgkin’s lymphoma can this be inaccurate? 2 years after having implants a large mass on neck popped up. 24 year old female just diagnosed with Hodgkin’s lymphoma two months after having baby and two years after implants
I was diagnosed with this in 2016 had removal of implants and one capsule I am from the uk and currently under Broomfield Hospital
Hi Linzi,
Thank you for sharing your diagnosis. What were your symptoms? What manufacturer and type of implant (smooth or textured) did you have? There is a Facebook support group called ALCL in Women with Breast Implants BIA-ALCL with 60+ other women who have been diagnosed with ALCL.
I had been poorly for long time and not connected it lots of colds fatigue aches pains for years then 2016 swollen right breast I had the mcghan implants still under th hospital for regular check ups
Hi Linzi, how did you get a diagnosis and who removed them? I am in the UK and the NHS refuse consultations and even a scan. I had a private surgeon write to them stating that they fitted my implant because I was lopsided, so I am their patient and my breathing is too bad for him to operate on in his clinic which they choose to ignore. Thanks